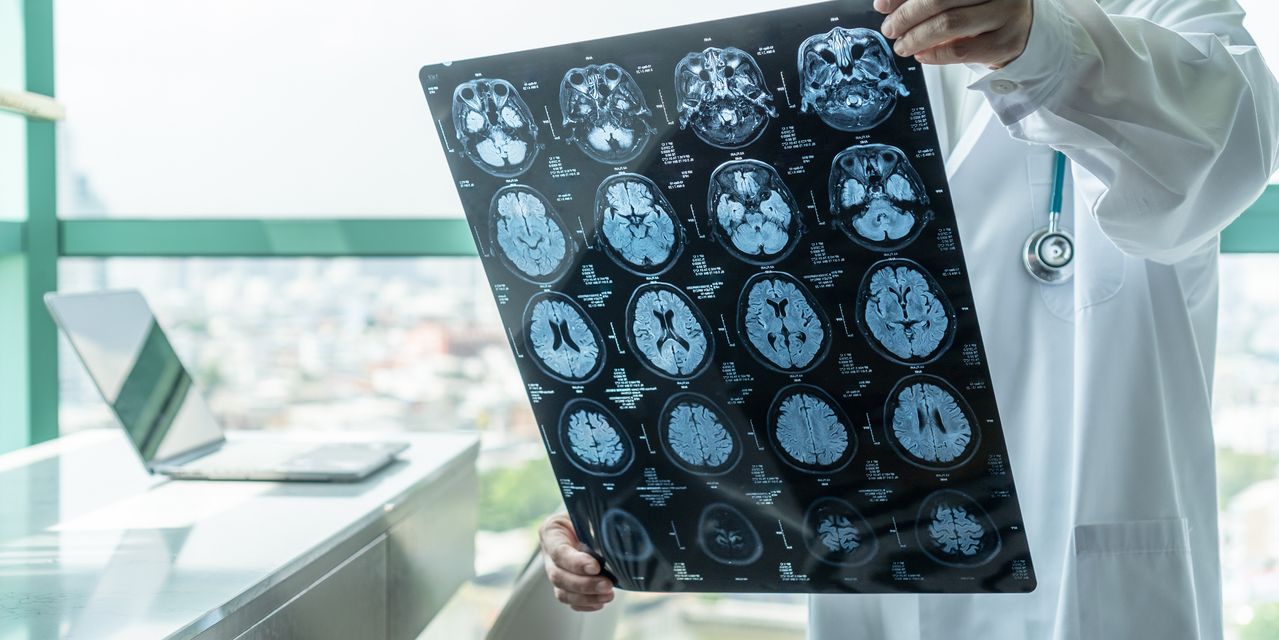
: Amid criticism for its handling of another Alzheimer’s drug, FDA will decide this week whether to approve lecanemab
A damning new congressional report found the regulator didn’t follow the correct procedures when it approved Aduhelm in 2021

Boston Fed President Susan Collins said Tuesday that there are risks to the U.S. economy not only from sticky inflation,...
Boeing’s credit ratings at heightened risk of downgrade to junk as strike puts company’s recovery “at risk,” S&P says.
U.S. consumers won’t see much of a change in the cost to heat their homes this winter compared to last...
Billionaire Mark Cuban has told Vice President Kamala Harris that one of her first priorities as president should be to...
Presidential candidate Kamala Harris says family caregivers need more help.
A chart of Microsoft’s stock will soon flash a clearly visible bearish signal that’s been in the works for the...